Day 1 :
Keynote Forum
Amarjit Bakshi
Refining Hydrocarbon Technologies LLC, USA
Keynote: Oil and gas developments and future growth
Time : 10:05-10:45

Biography:
Over 40 years’ experience in Engineering/ Consulting Management at senior level in Process Engineering, Technology, Business Development, Licensing, Acquisitions, Alliances and Project Management and Engineering, Operations Management and Process Engineering. Provided proven leadership and vision with broader perspectives and able to manage multiple tasks and personnel on mega projects.
Patents provide refiners and petrochemical plants innovations to enhance the performance of the units. Worked in all EU countries including UK, Germany, The Netherland. Major developments in Oil and gas business, downstream and petrochemicals technology, Catalysts, international alliance, licensing & contract negotiation, technology marketing, new technology commercial launch, partner relations. Dr Amarjit Bakshi has a Ph.D and also undergraduate degree both in Chemical Engineering from University of Surrey, Guildford, UK
Abstract:
In last 10 years major developments have changed the market where Middle East essentially Saudi Arabia was controlling the market. With the new developments in horizontal drilling and fracturing tight oil and gas formations have made major changes and USA has become exporter of oil and gas.
The prices have tumbled from 90+ dollars (which touched for a which to 147 dollars) to around 50 dollars per barrel (and touched below 30 dollars /barrel). This has happened due to onshore drilling in tight geological formations where one was able to recover gas and oil and with new technology of horizontal drilling one can recover oil/gas in 5 mile radius with one well which previously needed 20 wells. This provided at least better than breakeven economics around 40 to 50 dollars per barrel and gas is also very low cost and export of LNG from USA to Far East and Europe has started to meet the demand.
New developments provide much higher recovery of oil OOIP (original oil in place) and gas based on patent with moderate temperature and pressure CO2 and H2O2 in Classical EOR mode under critical conditions. Figure provides some information.
The future of oil and gas industry is going to be cyclic as before but there are some shifts taking place due to environment issues and new developments and growth in near term is going to be slow. Major issues are that diesel vehicles are being phased on coming years and electric cars and hybrid will take market share. So refinery operation will shrink due market place shift due to electric cars. The Oil and gas will move to petrochemicals and power production so shift will be there. Next 10 to 20 years will be challenging in developments in this area.

Keynote Forum
Denis Spitzer
Director NS3E Laboratory, France
Keynote: Spray Flash Evaporation (SFE): A technique for breakthrough chemical engineering challenges
Time : 11:05-11:45

Biography:
Dr. Habil. Denis SPITZER received his PHD in physical chemistry in 1993 at the University Louis Pasteur of Strasbourg. He is the founding and current Director of the NS3E Research Laboratory UMR 3208 ISL/CNRS/UNISTRA. He conducts research in continuous nanoscristallization processes of organic nanomaterials such as model medicaments and energetic materials. He is the inventor of the SFE process. He is the author of more than 150 publications and scientific reports. He received in 2013 the award of strategic thinking given by the French Homeland Minister, and more recently, in 2015, the « Grand Prix Lazare Carnot » award of the French Academy of Science, for dual use research.
Abstract:
The Spray Flash Evaporation (SFE) is a novel, innovative technique invented at the French-German research Institute of Saint-Louis (ISL). It permits to tailor and to engineer in a continuous way, organic, hybrid and inorganic nanomaterials in terms of particle sizes, crystallinity, structure and morphology from the laboratory scale to semi-industrial scale. It consists in the continuous spraying of a precursor (or a mixture of precursors), dissolved in an adapted solvent (or mixture of solvents) and heated at temperatures ranging from 130 °C to 160 °C at a pressure around 40 bar, followed by a rapid expansion through a hollow cone nozzle into an atomization chamber (20-50°C, 2-20 mbar. Due to the pressure difference, a very fast crystallization is induced, leading to the formation of nanoparticles, which are trapped by cyclones, filters or electrostatic precursors. This process allows the continuous nanocrystallizing (without any further thermal treatments) of organic, hybrid and inorganic crystallized pure compounds or nanocomposites. In the case of organic mixtures, depending on the kind of intermolecular interactions (Hydrogen bonds, π-π stacking, van der Waals interactions), the SFE technique is able to produce crystalline nanocomposites with dissociated or coupled compounds (core-shell), semi-amorphous or totally amorphous nanocomposites, and even, in the case of strong intermolecular interactions, nanococrystals. For example, in the latter case, different works showed the possibility to obtain nanococrystals such as Caffeine/Oxalic acid (2/1), Caffeine/Glutaric acid (1/1) and Resveratrol/4-aminobenzamid (1/1). The particle sizes, compared to them obtained by processes such as for example classical spray drying, are much smaller and are in this manner very important to enhance dissolution kinetics of drugs, additionally, to increase their bioavailability by the production of Active Pharmaceutical Ingredients (API) based on very small nanococrystals. Elaborating new types of molecular mixtures is also an upcoming challenge of SFE.
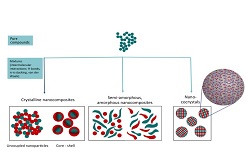
- Session 1: Chemical Engineering | Catalysis Engineering
Location: Sunset 2

Chair
Denis Spitzer
Director, NS3E Laboratory, France
Session Introduction
Amarjit Bakshi
Refining Hydrocarbon Technologies LLC, USA
Title: An overview of renewable fuels ethanol from cellulose and bio-diesel from conventional/algae feed status and economic options for ETBE
Time : 11:45-12:10

Biography:
Over 40 years’ experience in Engineering/ Consulting Management at senior level in Process Engineering, Technology, Business Development, Licensing, Acquisitions, Alliances and Project Management and Engineering, Operations Management and Process Engineering. Provided proven leadership and vision with broader perspectives and able to manage multiple tasks and personnel on mega projects.
Patents provide refiners and petrochemical plants innovations to enhance the performance of the units. Worked in all EU countries including UK, Germany, The Netherland. Major developments in Oil and gas business, downstream and petrochemicals technology, Catalysts, international alliance, licensing & contract negotiation, technology marketing, new technology commercial launch, partner relations.
Dr Amarjit Bakshi has a Ph.D and also undergraduate degree both in Chemical Engineering from University of Surrey, Guildford, UK
Abstract:
Advances in Biofuel technology: RHT-ETBE and RHT-TAEE are the Smart configuration technologies to enhance the conversion to over 97 to 90 percent respectively by having multiple side draws from the columns, and one can much better quality also than competitive technologies. The major advantage in these processes is that it allows wet ethanol to be use in the process and still meeting TBA and TAA specifications in the product. Essentially process is rejecting the water from wet ethanol and makes high quality Ethers at low Capex and Opex to the competitive processes. RHT- Biodiesel process is optimized to produce biodiesel from palm oil, Rape seed oil, vegetable and animal product that are all fatty acids with even number of carbon atom typically 12 to 22 atoms. This biodiesel is comparable to hydrocarbon diesel. The triglycerides are reacted with methanol/ ethanol or higher alcohol which all produce biodiesel in the acceptable boiling range. Methanol is most commonly used for the biodiesel production as being the cheapest alcohol, hence provides better economics. After the transesterfication reaction the product, methyl esters of those oils /fats as product and glycerine is produced as a byproduct. Glycerine is separated from the methyl esters of vegetable oils that are the biodiesel by phase separation by gravity settling due to density differences. The methyl esters and glycerine are purified to meet the product specifications. The technology is able to provide that reaction also to meet high overall conversions and selectivity at low Capex and Opex without producing any liquid waste.
Stephanie D. Lambert
University of Liege, Belgium
Title: Reforming of toluene with bimetallic catalysts supported on alumina and synthesized by an aqueous sol-gel process
Time : 12:10-12:35

Biography:
Stéphanie D. Lambert (SL) is a FRSâ€FNRS research associate and an associate professor in the Department of Chemical Engineering (DCE) of the University of Liege (Belgium) since 2009. She obtained her Ph.D. in Applied Sciences in 2003. After an engineer position in a Belgian chemical company (Nanocyl) (2004â€2005), and two postdoctoral stays at the DCE of the University of Illinois at Chicago in 2006, and at the Institute Charles Gerhardt in Montpellier in 2007, she joined the team “Nanomaterials, Catalysis, Electrochemistry” of the University of Liege, in which she develops heterogeneous catalysts for sustainable chemistry (tars reforming, treatments of chlorinated compounds, photocatalysis,..). She is viceâ€chair of the DCE since early 2016. SL has published over 75 publications, 12 book chapters, holds 1 patent and has an hâ€index of 18. She also received 14 Invited lectures. She is Member of Local Organizing Committee of SOL-GEL 2017, 3-8 septembre 2017, Liege, Belgium.
Abstract:
The thermochemical method called “biomass gasification” is generating emphatic interest for the production of bio-Syngas (CO+H2) since this process presents the advantage of being renewable without emitting CO2. However, in practical applications, there are still some technical problems due to high concentration of tars in the outlet gas, which can condensate and clog the pipes. Previous studies have highlighted the fact that the tar elimination via catalytic reforming seem to be the more practical and economical solution.
Catalysts were synthesized by an aqueous sol-gel process to develop γ-Al2O3 doped with 10wt.% of nickel and 2 wt.% of a second dopant (Co, Cu, Fe, Mn, Mo). Before their adding in AlOOH sol, metallic dopants were complexed with (OCH3)3-Si-(CH2)3-NH-(CH2)2-NH2 (EDAS) to increase their dispersion by cogelation between EDAS and AlOOH clusters.
All the samples were tested for toluene reforming: 31 vol.% CO, 31 vol.% H2, 15,5 vol.% CO2, 11 vol.% H2O, 9 vol.% CH4 and 24.000 ppm of toluene. The total flowrate was equal to 50 mL min-1. The temperature was set at 650°C for 300 min. No previous reduction step has been realized. Each 15 min, injection was sent to a GC Compac for analysis.
Figure 1 presents the toluene conversion as a function of benzene selectivity and as a function of carbon deposit after catalytic test for all the samples. For samples Al2O3-10Ni-2Mn and Al2O3-10Ni-2Mo, the addition of Mn or Mo allows increasing the toluene conversion up to 60%, whereas all other samples present lower toluene conversion (around 30%). Taking into account the benzene selectivity, it is observed that Mn and Mo are both elements that favor the degradation of aromatic groups. In term of carbon deposit during catalytic test, sample Al2O3-10Ni-2Mn is the most interesting doping since only 0.04 gcarbon gcata-1 is depicted by TG-DSC measurement after catalytic test.
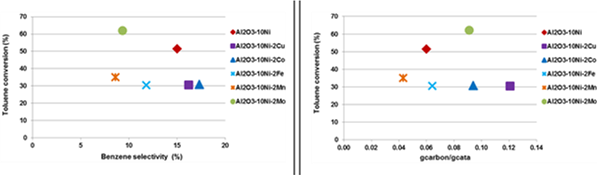
Figure 1: Toluene conversion as a function of the benzene selectivity and carbon deposit amount for all catalysts.
Emad Alhseinat
Khalifa University, UAE
Title: Foaming in gas sweetening process: Comprehensive experimental efforts lead to better understanding and predication of amine foaming
Time : 12:35-13:00

Biography:
Dr. Alhseinat is currently an Assistant Professor of Chemical Engineering at Khalifa University. Prior to join Khalifa University, Dr. Alhseinat completed his PhD from the University of Edinburgh. Then he worked in Abu Dhabi Petroleum Institute as Research and Teaching Associate; where he was heavily involved in research activity, writing and preparing scientific proposals and presentations, and publishing scientific articles. His current research activities address the development of novel separation processes compatible with renewable energy i.e. Magnetic nanoparticles, Electrical and Magnetic separation technologies, Foaming predication and monitoring, thermodynamics modelling and thermophysical properties characterization, Desalination and Water treatment, and Fouling science.
Abstract:
Comprehensive experimental work has been carried out to investigate the foaming behavior of aqueous Methyldiethanolamine (MDEA) in presence of twenty different contaminates including degradation products i.e. N,N,N-tris-(hydroxyethyl) ethylenediamine (THEED), hydroxyethyl ethylenediamine (HEED), N,N/-bis-(hydroxyethyl) piperazine (bHEP), N,N-bis-(2-hydroxyethyl) glycine (bicine), organic acids, and liquid organics. This foaming study was combined with physical characterization of the tested solution to enhance the understanding of the foaming behavior. The foaming tendency of aqueous MDEA solution was reported in terms of foam volume. Foam stability was reported on the basis of the time required for the last bubble to break. The results of this study showed that each contaminate has influenced the foaming behavior either by changing the foam volume or breaking time or both. However, it has been noticed that whatever is the added contaminates to the amine solution it drags the physical properties of the amine to a point where the foaming behavior will be changed. For example, in case of THEED and HEED, the addition of these degradation products increased the foam tendency and stability of the solution as a result of increasing solution viscosity; higher bulk viscosity retards the foam collapse caused by gravity drainage. It is believed that the bottleneck of predicating the foam behavior of any solution would be the predication and monitoring of its physical properties behavior and interaction. We are working now to develop the understating of the interaction between the physical properties and their combined effect on the foaming behavior of the amine solution; this will lead to a breakthrough in foaming monitoring and prediction. Mathematical models on tendency and stability of foaming are presented in this paper to explain the effect of physical properties on foam volume and breaking time of aqueous MDEA solutions.
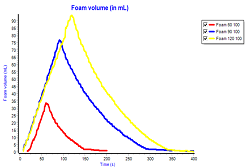
Fig. 1. Effect of time of foaming on foam volume for 0.1 wt. % BHCL with 50 wt. % MDEA solution at 100 ml/min.
Sinan Yapici
Inonu University, Turkey
Title: Effect of initial salt concentration on acid and base production from NH4Cl - NaCl solution by bipolar membrane electrodialysis
Time : 14:00-14:25

Biography:
Sinan YAPICI has his expertise in convective heat and mass transfer, electrodialysis applications and adsorption. He worked on electrochemical mass transfer in limiting current conditions, methods of enhancing convective heat and mass transfer, and adsorption of heavy metals, and applications of electrodialysis for synthesis of some inorganic acid and bases. He has got his PhD form Exeter university in UK, and has been lecturing in the university for more than 25 years.
Abstract:
In this study, a four-compartment bipolar membrane electrodialysis process was applied to produce ammonium hydroxide, sodium hydroxide and hydrochloric acid from an aqueous salt solution consisted of ammonium chloride and sodium chloride mixture. One of the most effective parameters in this sort of systems is the initial salt concentration. The purpose of this work is to study the effects of the initial ammonium chloride and sodium chloride concentration on the produced amount of hydrochloric acid, ammonium hydroxide and sodium hydroxide, the product-based salt conversion, the ion mass fluxes transferred through the membranes. The ratio of NH4CI/NaCI was kept constant and the initial values of NH4CI/NaCI ranged between 10/5 and 120/60 g/L. The other working conditions was maintained constant at the temperature of 25oC, electrolyte flow rates of 10 L/h, at the potential difference of 10 V and the initial acid and base concentration of 0.4 M. The experiments showed that the mass flux of the ions for the initial salt concentrations up to 40/20 (NH4CI/NaCI) g/L, first increased, and then started to decrease after reaching at a maximum. Above this value, the mass flux values decreased with time, having almost a constant value for a while and then continued to decrease again. These behaviour shows that the initial salt concentration has an important effect on the mass flux of ions, that is, the rate of the process. The conversion ratios up to a value of %70 was attained at the lowest initial salt concentration in a quite short processing time in about 100 minutes. As the salt concentration increased, the conversion reduced down below %50 for the highest salt concentration in a longer period of 480 minutes, showing the initial salt concentration has an important effect on conversion ratio. The consumed energy decreased with increasing initial salt concentration, from 2.22 to1.18 kWatt∙h/kg converted salt.
Acknowledgement: Thanks to TUBITAK for its support for this work with the project no: 115Y342
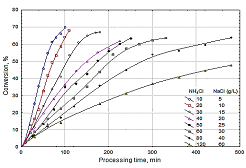
Figure 1. Change of conversion with initial salt concentration.
Jozsef Kupai
Budapest University of Technology and Economics, Hungary
Title: Synthesis and application of β-cyclodextrin-based cinchona organocatalysts and their recovery by organic solvent nanofiltration
Time : 14:25-14:50

Biography:
Jozsef Kupai focuses on the synthesis of enantioselective catalysts bearing cinchona alkaloid moieties. His research group applies cinchona-thioureas and squaramides in Michael additions with excellent yields and enantioselectivities. It was necessary to find different methods for the recovery of these catalysts to reach their sustainable application. Therefore, immobilization of cinchonas to different solid supports were achieved. On this conference, he will demonstrate size enlarging methods of cinchona catalysts to make them appropriate for recovery by OSN technique.
Abstract:
Organocatalysis, in which organic molecules catalyze single or multiple chemical transformations, has emerged as an efficient solution for the rapid and stereoselective synthesis of enantiomerically enriched molecules. Due to the many advantages of organocatalysis compared to conventional metal catalysis, organocatalytic methodologies have become an attractive synthetic tool in asymmetric catalysis.
Cinchona alkaloids and their derivatives have proven to be powerful organocatalysts owing to their reactivities, leading to high enantioselectivities. The presence of tunable functional groups enables cinchona alkaloids to catalyze a broad range of chemical reactions. Chiral thioureas and squaramides are promising classes of cinchona-based organocatalysts. Based on non-covalent interactions they behave as very efficient, directional hydrogen-bond donors.
Nowadays more and more attention is paid to protect our environment, and catalyst recovery can be a useful tool to reach this goal. Organic solvent nanofiltration (OSN) is an emerging technology that allows separation of solutes between 50 and 2000 g•mol-1 via pressure gradient in organic media. Attaching cinchona moieties to β-cyclodextrins (see Figure 1.) seems to be an effective way to reach convenient organocatalysts with high MW, leading to easy separation of the catalyst from the reaction mixture.
New β-cyclodextrin-cinchona thiourea and squaramide organocatalysts were prepared starting from commercially available β-cyclodextrin and hydroquinine. These bifunctional organocatalysts were applied successfully (up to 95% yield and 99% e.e.) in asymmetric Michael additions between 1,3-dioxo compounds and β-nitrostyrene.
After the enantioselective reactions, separation of the products and the CD-based catalysts were carried out applying the OSN method with high efficiency resulting in a green and sustainable organocatalytic method.
This work was supported by the Hungarian Scientific Research Fund/National Research, Development and Innovation Office [OTKA 112289, PD108462].
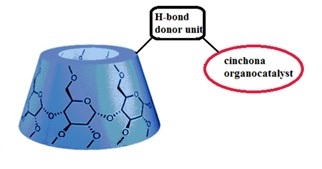
Figure 1. General structure of the new cyclodextrin-cinchona organocatalyst family.
José Angel Loredo-Medrano
University of Nuevo Leon, Mexico
Title: Mathematical model for the prediction of the dissolution of residual coffee beans for the sustainable production of energy materials
Time : 14:50-15:15

Biography:
José Angel Loredo-Medrano is a professor and researcher at the Autonomous University of Nuevo Leon in the Department of Chemical Engineering. He is active in the areas of process simulation and transportation phenomena. It belongs to several civil and academic associations.
Abstract:
The present work aims to establish and solve a model to predict the dissolution of the coffee beans to know the best time and concentration of solvent at room temperature. The mathematical model is based on the mass transport and chemical reaction phenomena considering the option of core in shrinkage and solved by applying the technique of orthogonal placement. The dissolution of more than 80% of the residual coffee grain in sulfuric acid at 60 ° C has been achieved. In order to make the process green, tests have been carried out with green solvents at room temperature, preferably looking for the starch. This is part of a research group project in which residual coffee grains are already used to remove heavy metals from wastewater. This new branch of the project seeks to obtain residues that allow totally clean energy and no residue from the dissolution process. The resolution of the model will allow to know the processes of dissolution, the distribution of products and predict the effect in the operating conditions. Each species is represented by a mass balance in which species diffusion and dissolution kinetics are included, the boundary conditions are: at the center of the particle the flow is zero and at the surface of the particle the convection from the surface to the bulk. To represent the shrinkage of the grain is represented the decrease of the radius with a total balance.
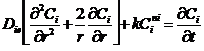
- Session 2: Biotechnology | Biologically Engineered Systems
Location: Sunset 2

Chair
Denis Spitzer
Director, NS3E Laboratory, France
Session Introduction
Jaron Hansen
Brigham Young University, USA
Title: Microbial pretreatment of biomass for renewable energy production
Time : 15:15-15:40

Biography:
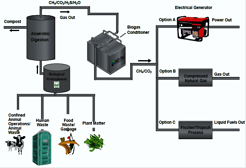
Jaron Hansen is a Professor of Chemistry and Biochemistry at Brigham Young University (Provo, Utah, USA) and co-founder of Verde and Anaerobic Digestion Technologies (AD Tec). His research involves improving the understanding of atmospheric and environmental chemical processes through focused laboratory, field and computational studies as well as the development of improved anaerobic digestion methods for enhanced production of biogas and for degradation of hazardous pollutants.
Abstract:
Without pretreatment, anaerobic digestion of lignocellulosic biomass typically converts only one-third of the carbon into biogas which is typically only 60% methane. Physical and chemical pretreatments to increase biogas production from biomass have proven to be uneconomical. The anaerobic thermophile, Caldicellulosiruptor bescii, has been shown to be capable of solubilizing up to 90% of lignocellulose, thus making the carbon accessible for anaerobic digestion. Preliminary experiments show C. bescii is capable of solubilizing a wide range of lignocellulosic materials. Anaerobic digestion readily and rapidly converts the soluble products into biogas with 70-80% methane. Isothermal biomicrocalorimetry measurements have provided a thermodynamic understanding of the process. We have applied the pretreatment-anaerobic digestion process to giant king grass and found the biogas yield significantly improved. Biomass Energy Solutions Technology, BEST, is currently collecting data on the pretreatment process with C. bescii and engineering system prototypes to prove feasibility for scale-up to megawatt facilities.
Conly Hansen
Utah State University, USA
Title: Biological breakdown of lignocellulose to produce renewable energy
Time : 15:40-16:05

Biography:
Dr. Conly Hansen has completed his PhD in Agricultural Engineering from The Ohio State University and joined as a Project Engineer for United States Army (discharged as Captain). At present, he is working as a Professor and Graduate Program Director at Center for Profitable Uses of Agricultural Byproducts, USA. He has published more than 56 research articles in reputed journals along with 6 book chapters; and presented more than 38 presentations with abstracts in national/international conference/symposia. He has around 14 significant honors on his name.
Abstract:
Lignocellulosic biomass is the most abundantly available raw material on the Earth for the production of biofuels. The conversion of lignocellulose into renewable energy and more valuable chemicals has been limited. Several methods for increasing the conversion of lignocellulose into biogas by pretreating the feedstock have been developed, but all of the existing methods have large economic penalties, e.g. disposal of toxic wastes and greatly increased capital and operating costs. The discovery and characterization of Caldicellulosiruptor microbes, extremophilic organisms capable of solubilizing lignocellulose, suggested a possible solution to the economic problem of pretreatment. For example, researchers (Blumer-Schuette et al., 2014) have found that up to 90% of the biomass in switchgrass could be solubilized by C. bescii, thus providing proof of concept.
Beginning in 2014, recognizing the potential for anaerobic digestion of lignocellulose for biogas production, a multidisciplinary team including a biochemist, chemist, microbiologist and agricultural engineer, from Brigham Young and Utah State Universities has been conducting experiments to determine if we could break down other lignocellulose feedstocks for later anaerobic digestion. Our experimental results have been very encouraging in providing proof of concept that anaerobic digestion of lignocellulose, something that has never been done in a commercially viable way, may be both possible and attainable. However, the commercialization and scaling up of lab experiments is not simply a process of buying bigger tanks but requires cross-discipline expertise in order to overcome the inevitable differences between 20-1000 mL lab experimentation and a multiple m3 system. This presentation will report the results of work we have done to take the process from the lab to the market; the hurdles to scaling and commercializing the anaerobic digestion of lignocellulose in an economically viable way.
Kenji Abe
Ajinomoto Co. Inc., Japan
Title: Engineering of Escherichia coli to facilitate efficient utilization of isomaltose and panose in industrial glucose feedstock
Time : 16:25-16:50

Biography:
Kenji Abe is an employee of Japanese company, Ajinomoto Co., Inc.. He has his expertise in industrial fermentation technology and biotechnology.
Abstract:
Fermentative production of useful compounds, such as alcohols, gases, pharmaceutical ingredients, and organic/amino acids, is conducted worldwide. Escherichia coli is one of the most useful bacterium for the production of valuable compounds, such as amino acids and organic acids, because E. coli cells grow quickly, convert substrates to products rapidly, and are genetically engineered readily. For example, L-lysine, which is used as a feed additive worldwide, is produced on the scale of approximately 1,500,000 metric tons per year.
On the other hand, in the industrial production of useful compounds by fermentation, glucose is one of the most frequently used carbon sources. Industrial glucose feedstock prepared by enzymatic digestion of starch typically contains significant amounts of disaccharides such as maltose and isomaltose, and trisaccharides such as maltotriose and panose. Maltose and maltosaccharides can be utilized in Escherichia coli fermentation using industrial glucose feedstock because there is an intrinsic assimilation pathway for these sugars. However, saccharides that contain α-1,6 bonds, such as isomaltose and panose, are still present after fermentation because there is no metabolic pathway for these sugars. To facilitate more efficient utilization of glucose feedstock, we introduced glvA, which encodes phospho-α-glucosidase, and glvC, which encodes a subunit of the phosphoenolpyruvate-dependent maltose phosphotransferase system (PTS) of Bacillus subtilis, into E. coli. The heterologous expression of glvA and glvC conferred upon the recombinant the ability to assimilate isomaltose and panose. The recombinant E. coli assimilated not only other disaccharides but also trisaccharides, including alcohol forms of these saccharides, such as isomaltitol. To the best of our knowledge, this is the first report to show the involvement of the microbial PTS in the assimilation of trisaccharides. Furthermore, we demonstrated that an L-lysine-producing E. coli harboring glvA and glvC converted isomaltose and panose to L-lysine efficiently. These findings are expected to be beneficial for industrial fermentation.
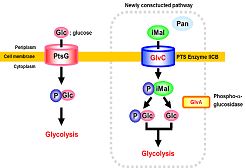
Figure 1. Schematic illustaration of GlvAC pathway for assimilation of isomaltose (iMal) and panose (Pan).
